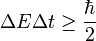One form of energy can often be readily transformed into another with the help of a device- for instance, a battery, from chemical energy to electric energy; a dam: gravitational potential energy to kinetic energy of moving water (and the blades of a turbine) and ultimately to electric energy through an electric generator. Similarly, in the case of a chemical explosion, chemical potential energy is transformed to kinetic energy and thermal energy in a very short time. Yet another example is that of a pendulum. At its highest points the kinetic energy is zero and the gravitational potential energy is at maximum. At its lowest point the kinetic energy is at maximum and is equal to the decrease of potential energy. If one (unrealistically) assumes that there is no friction, the conversion of energy between these processes is perfect, and the pendulum will continue swinging forever.
Energy gives rise to weight and is equivalent to matter and vice versa. The formula E = mc², derived by Albert Einstein (1905) quantifies the relationship between mass and rest energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by J. J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass-energy equivalence#History for further information). Since c2 is extremely large relative to ordinary human scales, the conversion of ordinary amount of mass (say, 1 kg) to other forms of energy can liberate tremendous amounts of energy (~9x1016 joules), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (particles) are found in high energy nuclear physics.
In nature, transformations of energy can be fundamentally classed into two kinds: those that are thermodynamically reversible, and those that are thermodynamically irreversible. A reversible process in thermodynamics is one in which no energy is dissipated (spread) into empty energy states available in a volume, from which it cannot be recovered into more concentrated forms (fewer quantum states), without degradation of even more energy. A reversible process is one in which this sort of dissipation does not happen. For example, conversion of energy from one type of potential field to another, is reversible, as in the pendulum system described above. In processes where heat is generated, quantum states of lower energy, present as possible exitations in fields between atoms, act as a reservoir for part of the energy, from which it cannot be recovered, in order to be converted with 100% efficiency into other forms of energy. In this case, the energy must partly stay as heat, and cannot be completely recovered as usable energy, except at the price of an increase in some other kind of heat-like increase in disorder in quantum states, in the universe (such as an expansion of matter, or a randomization in a crystal).
As the universe evolves in time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or other kinds of increases in disorder). This has been referred to as the inevitable thermodynamic heat death of the universe. In this heat death the energy of the universe does not change, but the fraction of energy which is available to do produce work through a heat engine, or be transformed to other usable forms of energy (through the use of generators attached to heat engines), grows less and less.
Law of conservation of energy
Main article: Conservation of energy
Energy is subject to the law of conservation of energy. According to this law, energy can neither be created (produced) nor destroyed by itself. It can only be transformed.
Most kinds of energy (with gravitational energy being a notable exception)[20] are also subject to strict local conservation laws, as well. In this case, energy can only be exchanged between adjacent regions of space, and all observers agree as to the volumetric density of energy in any given space. There is also a global law of conservation of energy, stating that the total energy of the universe cannot change; this is a corollary of the local law, but not vice versa.[8][13] Conservation of energy is the mathematical consequence of translational symmetry of time (that is, the indistinguishability of time intervals taken at different time)[21] - see Noether's theorem.
According to energy conservation law the total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system.
This law is a fundamental principle of physics. It follows from the translational symmetry of time, a property of most phenomena below the cosmic scale that makes them independent of their locations on the time coordinate. Put differently, yesterday, today, and tomorrow are physically indistinguishable.
This is because energy is the quantity which is canonical conjugate to time. This mathematical entanglement of energy and time also results in the uncertainty principle - it is impossible to define the exact amount of energy during any definite time interval. The uncertainty principle should not be confused with energy conservation - rather it provides mathematical limits to which energy can in principle be defined and measured.
In quantum mechanics energy is expressed using the Hamiltonian operator. On any time scales, the uncertainty in the energy is by
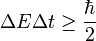
which is similar in form to the Heisenberg uncertainty principle (but not really mathematically equivalent thereto, since H and t are not dynamically conjugate variables, neither in classical nor in quantum mechanics).
In particle physics, this inequality permits a qualitative understanding of virtual particles which carry momentum, exchange by which and with real particles, is responsible for the creation of all known fundamental forces (more accurately known as fundamental interactions). Virtual photons (which are simply lowest quantum mechanical energy state of photons) are also responsible for electrostatic interaction between electric charges (which results in Coulomb law), for spontaneous radiative decay of exited atomic and nuclear states, for the Casimir force, for van der Waals bond forces and some other observable phenomena.
Energy and life
Main article: Bioenergetics

Basic overview of energy and human life.
Any living organism relies on an external source of energy—radiation from the Sun in the case of green plants; chemical energy in some form in the case of animals—to be able to grow and reproduce. The daily 1500–2000 Calories (6–8 MJ) recommended for a human adult are taken as a combination of oxygen and food molecules, the latter mostly carbohydrates and fats, of which glucose (C6H12O6) and stearin (C57H110O6) are convenient examples. The food molecules are oxidised to carbon dioxide and water in the mitochondria
-
- C6H12O6 + 6O2 → 6CO2 + 6H2O
- C57H110O6 + 81.5O2 → 57CO2 + 55H2O
and some of the energy is used to convert ADP into ATP
-
- ADP + HPO42− → ATP + H2O
The rest of the chemical energy in the carbohydrate or fat is converted into heat: the ATP is used as a sort of "energy currency", and some of the chemical energy it contains when split and reacted with water, is used for other metabolism (at each stage of a metabolic pathway, some chemical energy is converted into heat). Only a tiny fraction of the original chemical energy is used for work:[22]
- gain in kinetic energy of a sprinter during a 100 m race: 4 kJ
- gain in gravitational potential energy of a 150 kg weight lifted through 2 metres: 3kJ
- Daily food intake of a normal adult: 6–8 MJ
It would appear that living organisms are remarkably inefficient (in the physical sense) in their use of the energy they receive (chemical energy or radiation), and it is true that most real machines manage higher efficiencies. In growing organisms the energy that is converted to heat serves a vital purpose, as it allows the organism tissue to be highly ordered with regard to the molecules it is built from. The second law of thermodynamics states that energy (and matter) tends to become more evenly spread out across the universe: to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe ("the surroundings").[23] Simpler organisms can achieve higher energy efficiencies than more complex ones, but the complex organisms can occupy ecological niches that are not available to their simpler brethren. The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ecology: to take just the first step in the food chain, of the estimated 124.7 Pg/a of carbon that is fixed by photosynthesis, 64.3 Pg/a (52%) are used for the metabolism of green plants,[24] i.e. reconverted into carbon dioxide and heat.


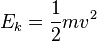 (half mass times velocity squared). Then the total amount of energy can be found by adding
(half mass times velocity squared). Then the total amount of energy can be found by adding 
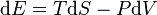 ,
, ,
,